
Take the emotion out of impactful market events by actively planning and remaining invested for the long term.
There’s been a lot of talk about the potential for a recession as of late with pundits warning that threatening clouds may be on the horizon. Recession is a term that strikes fear in the hearts of ordinary investors. Of course it’s easy to understand the adverse reaction, but market events can also provide a world of opportunity as well.
It’s Just a Phase
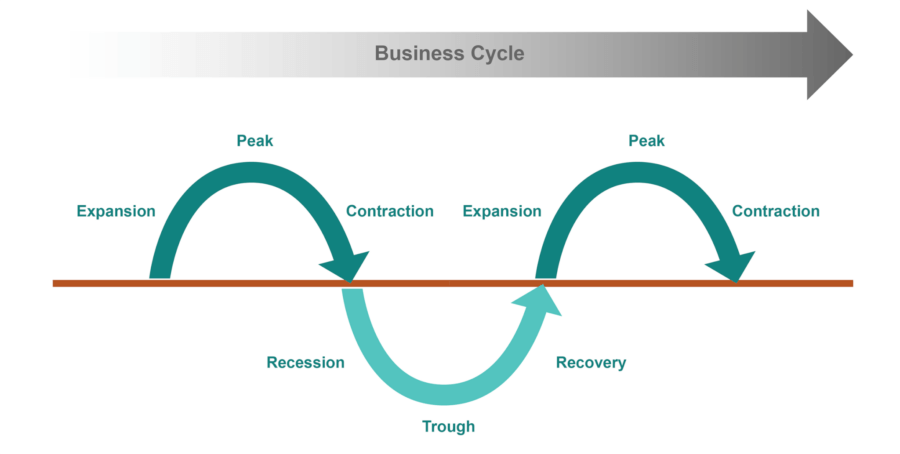
Let’s talk about recession. It can cause pain in the event of short-term job loss, but it also can be healthy for the economy over the longer-term. The media has positioned recessions as the monster lurking in the shadows, waiting for an opportunity to catch investors off guard. But instead of fearing the unknown, recessions are merely a part of the business cycle, the expansion and contraction of the economy, which is as natural as the four seasons. Business cycles have the following phases:
- An economic expansion is characterized by positive Gross Domestic Product (GDP) growth, low unemployment, a bull market, and consumer confidence.
- A business cycle peak is the transition from the end of the expansion to the contraction.
- During a contraction, consumer demand slows, the economy weakens, unemployment increases, and stock prices usually decline. A recession occurs when GDP growth has been negative for two consecutive quarters.
- A trough represents the transition from the contraction phase to the expansion phase.
Business Cycle
The line represents a demarcation for entering and exiting a recession
Cooling Changes
Despite all of the emotional noise, recessions are beneficial because they provide relief for an economy that has overheated. Recessions can also generate two positive changes in behavior:
- Consumers cut back on their spending and reevaluate their finances.
- Companies make a concerted effort to better manage their assets and workforce.
Instead of panicking at the very mention and thought of a recession, investors could be better positioned with an enhancement of knowledge. By gaining a better understanding and recognizing the inevitability of recessions, investors can plan for their eventuality and then greet the typical expansion that follows with open arms.
Planning Matters
Active planning is a critical step in the process because recessions often force investors to cut back and make difficult allocation decisions. One of the best ways to prepare is to establish an emergency fund. An emergency fund provides peace of mind and eliminates the need to sell investments into a declining market. While the desired amount of an emergency fund varies, most experts recommend setting aside an amount equal to at least six months of expenses in an investment that doesn’t dramatically fluctuate (like a money market or a low duration bond fund).
In reality, when stock prices are falling, that’s when you’d ideally want to take advantage of the opportunity and invest. If there isn’t extra money to put into the market, simply remaining invested can often pay off handsomely. For example, during the recession of 2008–2009, the S&P 500 Index hit a low in March 2009. In the 10 years between March 2009 and March 2019, the S&P 500 delivered a 10-year annualized total return of nearly 18%. Those investment returns amply rewarded investors who held steady through the emotional pain of that unprecedented period.
Key Takeaway
Once educated investors understand recessions, they won’t run the next time someone yells “Recession!” in a crowded theater. Instead, investors will see it as an opportunity to remain invested for the long term, which may ultimately lead to their financial well-being.
Discover more about:
More Insights

Real Estate Market Recap: Trends, Challenges, and What Comes Next

Beyond the Hype—Building Smarter Private Credit Portfolios

Structural Forces are Driving the Current Investment Environment

Key Principles of the ETF Execution Protocol
Finding Opportunity Amid Imbalance: 2026 Market Outlook
